Drug Development Process and Jobs in Pharmaceutical Industry

Basic research – target identification and validation
The R in R&D refers to basic research groundwork to discover disease mechanisms and potential drug targets that could be modulated to treat the disease. Key aspects of basic research include investigating disease biology at the molecular and cellular level to identify proteins, genes, or pathways involved. Another aspect involves identifying putative target molecules or pathways that could be drugged to produce a therapeutic effect through data mining of available literature, phenotype screening1 and bioinformatics (epigenetic, genomic, transcriptomic, and proteomic methods). These targets are then validated by various approaches (sense reversal like gene knockouts, antisense technology, and RNAi, proteomics to manipulate protein expression level)2 in vitro and in animal models to confirm its ability to produce the therapeutic benefit. Much of the research occurs in the public sphere like in academia funded by the government and philanthropic organizations (NIH and private disease foundations) as such knowledge may not be patentable, are widely disseminated and may not produce a profitable return for pharmaceutical companies (not all targets identified lead to a drug).3
Hit Discovery
Drug discovery is the first step in the development of new medicines. It involves screening of thousands of therapeutic agents (small molecules, biologics) to find ones that could be drug candidates to modulate the response of a selected target. This process requires the use of various experimental and computational tools to design (AI-aided drug design), synthesize (retrosynthesis algorithm), and test thousands to millions and billions of molecules via high-throughput screening using various assay methods in silico (virtual screening or molecular modeling, whole-cell simulations and ligand-based drug design)4, in vitro (colorimetric, fluorescence, bioluminescence, FRET, HPLC, ELISA, SPR, MS, fragment screening, NMR, and DNA encoded chemical libraries), and in vivo (cell-based immunofluorescence, reporter gene, mammalian two-hybrid, fluorescence-based IHC assays) and ex vivo (in tissues or organs).
Hit-to-Lead Phase
The search process for hit compounds can be iterative to identify structure-activity relationship and repeated to ensure replicability while examining dose-response curves at cellular and tissue levels to assure efficacy in complex systems compared to isolated target in initial assays, ease of chemical synthesis and potential for further modification for optimization. Assay development scientist is an important role in this area. After hit compounds are identified, their absorption, distribution, metabolism, excretion (ADME) and pharmacodynamics properties are studied and optimized to produce promising candidates for in vivo efficacy. Physiochemical and pharmacokinetic measurements and selectivity profiling are also carried out to paint a complete profile of the efficacy and side effects of the hits. A hypothetical screening process is shown in Fig. 1.5
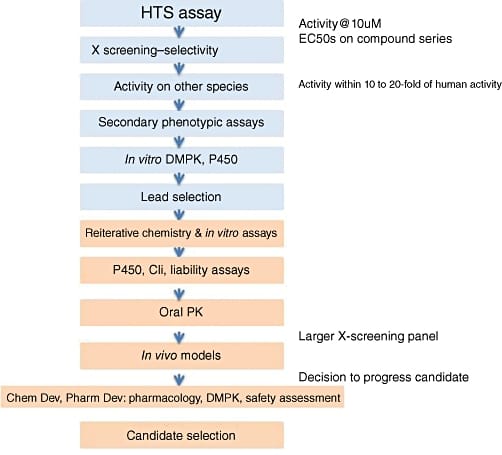
Fig 1: A hypothetical screening process from high throughput screening to candidate selection.5
Lead Optimization
The compounds at this stage undergoes further modification to its structures to optimize its drug metabolism and pharmacokinetic (DMPK) properties and address any issues identified in previous screenings. In the meantime, hit discovery continues to explore and fine-tune potential SAR series to find synthetically varied back-up molecules in case the lead molecules fail at the clinical phase.
Preclinical and Clinical Phases
Drug candidates then go through detailed testing to evaluate its safety and potential toxicity in animal models. Preclinical studies help researchers to design phase I studies, for example, determining maximum safe starting dose and identifying safety evaluation criteria based on animal model results following FDA’s guidance.6 The pharmacological profile of the candidates now can be used to develop initial manufacturing process and pharmaceutical formulation to be used for testing in clinical trials. Investigational New Drug (IND) application may be filed with preclinical results and once this is approved, clinical trials may begin. Clinical trials go through several phases with phase I evaluating drug safety, II being high-risk proof-of-concept study (IIa) and larger studies with comparator agents and broader dosages to determine correct dosing regimen and monitor side effects (IIb), and III being comprehensive large-scale studies to obtain regulatory approval. Phase III trials confirm efficacy and compare the drug candidates to commonly used treatments or placebo in ideally double-blinded manner in multi-centers involving thousands of patients. Successful trials lead to a New Drug Application (NDA) or Biologics License Application (BLA) submission to FDA. Phase IV trials may be conducted to study the drug’s long-term safety and effectiveness by collecting data from patients taking the medication in real-world settings through observational studies or randomized controlled trials as required by FDA or voluntarily by companies for marketing purposes after regulatory approval from the FDA.
Roles in Pharmaceutical R&D Space
Target identification: molecular biologist to study the disease mechanism and identify therapeutic targets, bioinformatics scientists or biostatisticians.
Hit discovery roles: design and synthesis drug entities (small molecule by chemists or biologics by molecular or synthetic biologists, data scientists for in silico screening).
Assay development: develop suitable assay methods to evaluate the effect of chemical compounds on targets.
Molecular and Cellular pharmacology: evaluate and model drug DMPK and pharmacodynamics properties.
Clinical trials: clinical research associate, clinical data manager, biostatistician to design, set up and analyze clinical studies.
Regulatory affairs: ensure compliance with regulatory standards.
Analytical sciences: quality assurance/quality control professionals to develop and implement quality standards and procedures to ensure adherence to CGMP.
Formulation and process development: design and develop formulation, scale-up and manufacturing process of drugs.
Reference
2. https://doi.org/10.1038/422341a
3. https://www.biospace.com/opinion-who-really-pays-for-drug-development-both-government-and-industry [Accessed Oct 3, 2024]
4. 10.3390/pharmaceutics15010049
5. 10.1111/j.1476-5381.2010.01127.x
6. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/estimating-maximum-safe-starting-dose-initial-clinical-trials-therapeutics-adult-healthy-volunteers [Accessed Oct 3, 2024]